WSAS is a multidisciplinary population-based longitudinal study on obstructive airway diseases and allergies in adults. WSAS started in 2008 and involves research from cellular mechanisms to clinical and population epidemiological studies.
Obstructive respiratory diseases, including asthma, COPD and allergies are among the major public health diseases. The research project, West Sweden Asthma Study (WSAS), studies the prevalence and clinical characteristics of asthma and allergies in a population setting. Our overarching aim is to characterize the burden of asthma, its risk and preventative factors, its phenotypes and causes in an adult-based population setting from the epidemiological, clinical, and mechanistic standpoints. The project has four sub-aims, including:
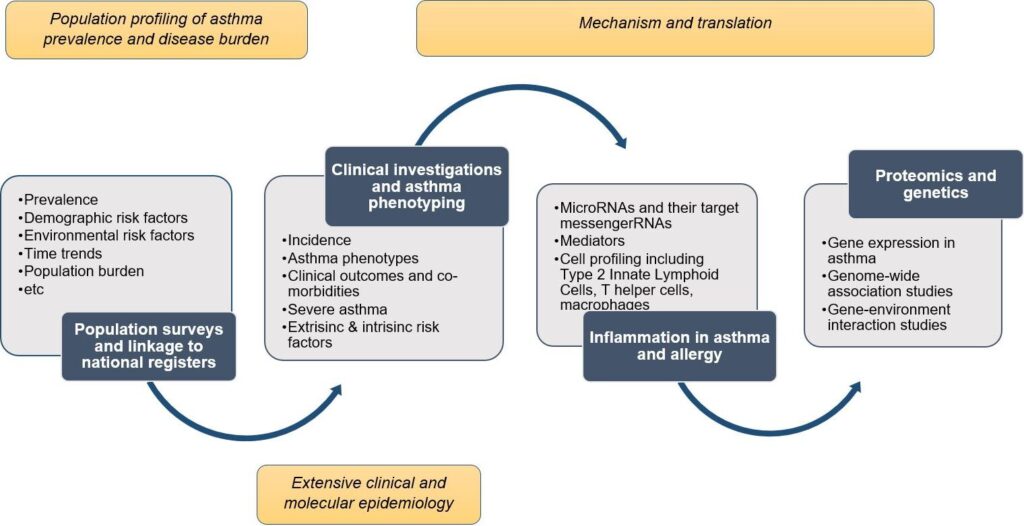
WSAS started in 2008 and includes both postal surveys and in-depth clinical investigations and structured interviews. In total, the postal questionnaire includes 42,621 adults aged 16-75 years, while the clinical investigations, currently ongoing, are aimed to include at least 8,000 participants who will undergo comprehensive clinical profiling.
The study includes several different groups of participants that are followed over time. Particular focus is currently on studies of severe asthma, the impact of sex hormones on asthma, and computational phenotyping of the diseases. Recently, we have also integrated investigations into long COVID, its pre-COVID determinants, and clinical and mechanistic aspects into WSAS. We have also linked data that we have collected with data from different registers. In addition to survey questionnaires and detailed clinical interviews conducted, other measurements performed in WSAS include, but not limited to, physical examinations, lung function measurements with dynamic spirometry, bronchial provocation test with methacholine, fraction of exhaled nitric oxide and measurement of various biological and blood samples, including allergy testing with specific IgE, and skin prick tests. Other planned measurements will include proteomics, transcriptomics, genomics, genetics. The COVID-19 follow-up study will include various respiratory, cardiovascular, neuro-psychologic, and immunological measurements
Within the project, we have our own research clinic with specially trained research nurses and biomedical analysts who meet hundreds of participants every year in the various projects that are conducted at Krefting Research Centre.
Anchor: Main sub-projectsAsthma together with allergic diseases are among the great diseases of our time and constitute the largest disease group among children and adults up to middle age. It is estimated that 300-400 million people worldwide have asthma. The prevalence of asthma has increased significantly in many parts of the world. For the dimension of care, it is important to know about how the prevalence of major diseases such as asthma changes over time. Changed environmental exposure and lifestyle can be important for incidence, disease progression, remission and relapse.
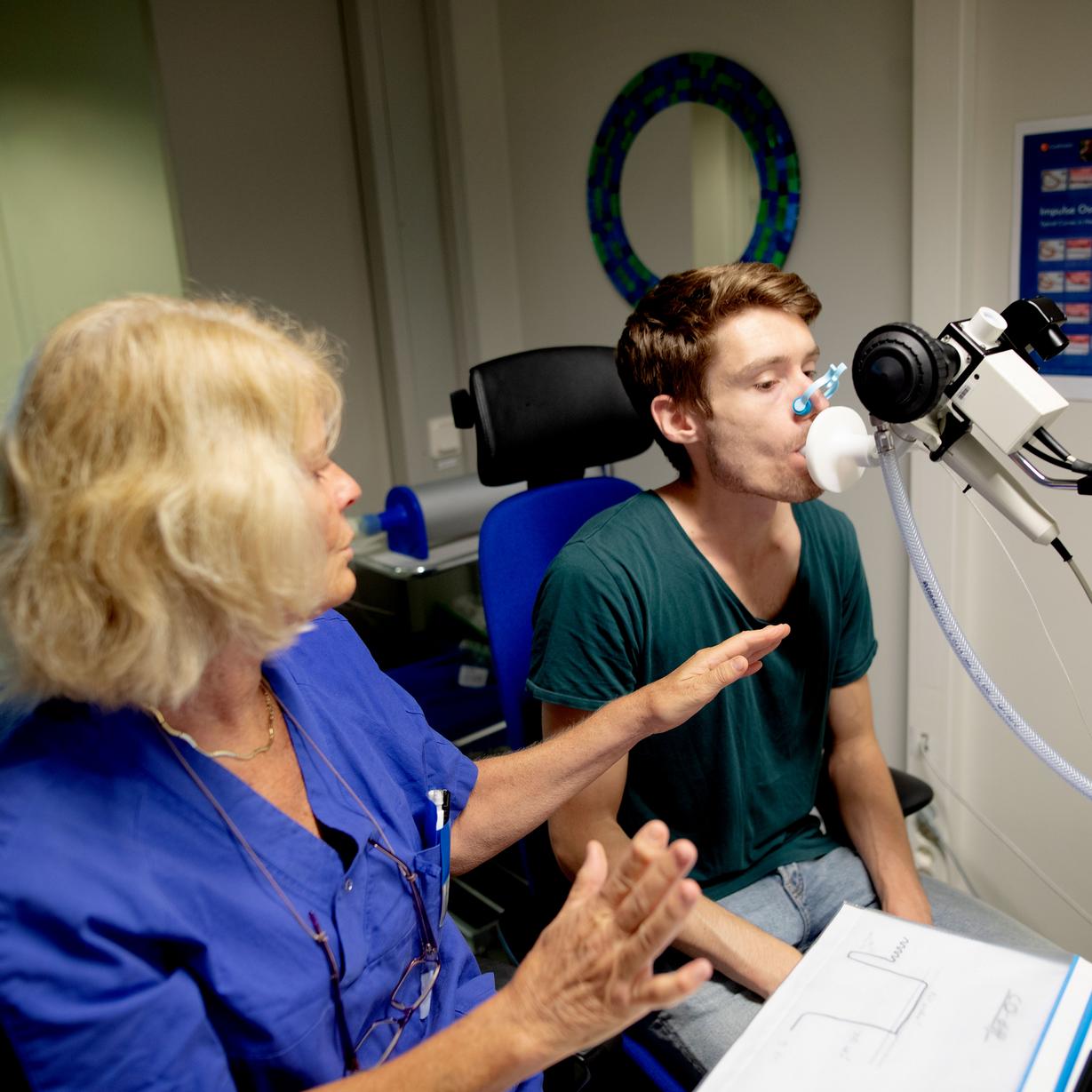
There is limited data on the prevalence and characteristics of severe asthma in the population. and we need to generate more knowledge about prevalence, clinical characteristics and treatment and the implications of having severe asthma. Severe asthma consists of several different phenotypes and how these should be treated is partly unclear. Knowledge of the development of incidence and severity of asthma is of great importance to society. The study provides a unique opportunity to gain knowledge from a population-based sample, knowledge that is limited today. Severe asthma leads to frequent health care visits and accounts for most of the health economic costs of asthma. To be able to develop interventions and reduce the burden of illness for people with severe asthma, further research is needed that focuses on clinical and health psychological aspects.
The study focuses on issues of severe asthma in an epidemiological setting. Particular focus will be on: prevalence and demographics of severe asthma according to current definitions; identification of impactable risk factors; the occurrence of anxiety and depression and its impact on health-related quality of life; knowledge about how people with severe asthma manage their illness and what support they need to reach their treatment goals.
Lina Rönnebjerg
Hannu Kankaanranta
Linda Ekerljung
An intriguing sex-related differences in asthma has been observed for decades, whereby asthma is more common in boys than in girls during early childhood, but becomes more common and more severe starting from puberty and into adulthood. While the cause of these gender-related differences are not fully understood, the female sex hormones are thought to largely play a role. The female’s life is characterized by several hormonal transition points – menarche, menstruation, pregnancy, and menopause – and each of these has been linked to asthma. The use of hormonal contraception during the reproductive life and hormone therapies during menopause have also been hypothesized to play a role in asthma in women. Although several studies have been performed during the last four decades to understand the role of the female sex hormones in the pathogenesis of asthma, the overall literature remains conflicting.
Within the West Sweden Asthma Study, our overarching aim is to definitively elucidate on the role of female sex steroids in the pathogenesis and clinical manifestation of asthma in women. In addressing this question, our investigations cover clinical and genetic epidemiology, from where we aim to undertake mechanistic studies to try to uncover the biological processes through which sex steroids influence asthma in women.
Guo-Qiang Zhang
Hannu Kankaanranta
Saliha Selin Özuygur
Madeleine Rådinger
Bright Nwaru
Current research advances have shown that obstructive airway diseases are heterogeneous conditions, both in their clinical manifestation and disease severity. This has persuaded current recognition that optimal treatment of these diseases can be attained when treatments are individualized. However, achieving an individual-tailored treatment requires a clear understanding of the phenotypic characteristics of each patient. Disease phenotyping will: (1) contribute to better understanding of the underlying disease pathogenesis; and (2) provide a clinical support for attaining individual tailored treatment. Computational approaches that use high-level computer programming have been particularly useful in uncovering the different phenotypes of human diseases.
In this project, based on the West Sweden Asthma Study and in collaboration with the Obstructive Lung Disease in Northern Sweden, we are using novel computational methods to identify distinct phenotypes of obstructive airway diseases in adults. We then investigate the impact of environmental and genetic factors on the derived phenotypes, as well as the healthcare use trajectories and co-morbidity patterns of the derived phenotypes.
Muwada Bashir
Daniil Lisik
Rani Basna
Lowie Vanfleteren
Hannu Kankaaranta
Bright Nwaru
Histamine intolerance (HIT) has been reported for several decades, but despite this, the knowledge of background and treatment is unclear, the exact prevalence is unknown but is estimated at about 1%. HIT’s pathophysiology is unclear but is believed to be a non-IgE mediated food hypersensitivity. Histamine and other bioactive amines are found in some foods and furthermore certain foods can release the body’s own histamine. There is relatively weak scientific evidence that hypersensitivity is partly due to decreased activity of diamine oxidase (DAO), which is the major enzyme involved in histamine degradation. There is no knowledge of how to clinically use and evaluate DAO in patients. Lactose intolerance is characterized by the complete or partially limited ability to break down the carbohydrate lactose that is found naturally in dairy products such as milk, fil, yogurt, cream. Recent years’ research has defined lactose as carbohydrate with increased fermentation capacity in people with functional gastrointestinal disorders, so-called IBS. The most defined cause of hypersensitivity to gluten-containing cereals is celiac disease. Celiac disease should be diagnosed in a doctor by checking antibodies in the blood and in most cases doing a small bowel biopsy. It has been discussed that diagnosis without biopsy can be made if there is a high concentration of transglutaminase antibodies. The number who choose gluten from the diet in the Nordic countries has limited knowledge of.
The study aims to determine the normal interval for DAO, if there is a pathological reference area and to identify factors that may influence DAO activity. The study is of clinical significance as the number of questions and referrals around suspected histamine hypersensitivity increases in adult allergy and in the community and on social media. However, knowledge about the problem of this hypersensitivity is very limited. Furthermore, the study aims to measure the prevalence of self- and physician-diagnosed gluten and lactose intolerance in the population, describe which symptom picture they suffer based on their self-diagnosis and determine if there is a link to other food sensitivities and allergies?
Adina Weisheit
Bright Nwaru
Linda Ekerljung
Collaboration with the Allergy Clinic at Sahlgrenska University Hospital chief physician Monica Arvidsson and dietitian Jenny van Odjik
Accurate diagnosis of allergy is essential in order to provide accurate estimates of its burden, as well as to provide appropriate, potentially life-saving therapy. Traditionally, the diagnosis of allergy has been typically based on self-report and/or combination of skin prick tests (SPT) or immunoassays of serum specific IgE levels. Whilst SPT and specific IgE, as objective measures, have reasonable sensitivity, they have sub-optimal specificity and are in general poorly predictive of the severity of reactions and cross-reactivity of allergens. During the last decade, the limitations of these conventional methods for diagnosing allergy have stimulated promising developments in molecular-based diagnostic techniques, collectively referred to as component-resolved diagnosis (CRD)
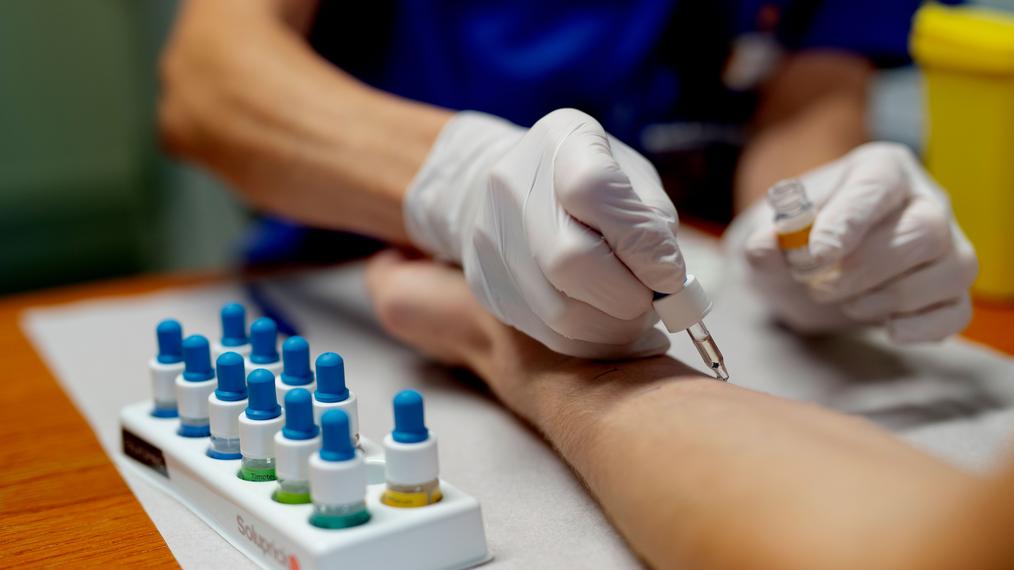
CRD involves examining the specific allergen proteins, primarily at the molecular level. In this case, rather than providing a summary of whole allergen extracts, CRD quantifies the individual allergen proteins that may be responsible for allergic reactions. In CRD, specific-IgE responses are evaluated against individual allergenic molecules or the epitopes of those allergens. CRD techniques therefore have the potential to enhance the specificity and sensitivity of serum specific-IgE responses to allergens. In addition to facilitating our ability to determine specific allergen phenotypes, CRD may help improve compilation of a patient-tailored risk profile to specific allergens, given that IgE antibodies to allergen molecules may vary from patient to patient. Finally, component-based IgE testing can distinguish a primary sensitization from a cross-sensitization to a higher extent than whole allergen extracts.
While several molecular allergen components to specific allergens have now been developed, most clinical and epidemiological studies characterizing these components and evaluating their impact on asthma rhinitis, and clinical outcomes have been performed in children. There is currently a paucity of data in adults. WSAS provides a unique setting to help advance knowledge of molecular in adults and evaluate their impact on clinical outcomes of asthma.
The overarching aim of this sub-study is to characterize molecular allergy in adults based on CRD and then investigate the influence of molecular allergen components in the development of new onset asthma and rhinitis and manifestation of clinical outcomes of asthma in adults.
Saliha Selin Özuygur
Rani Basna
Emma Goksör
Göran Wennergren
Jan Lötvall
Hannu Kankaaranta
Bright Nwaru
Asthma was traditionally considered to be a single, allergic, eosinophilic and type 2 inflammation (T2)-mediated disease (see project on “Immune mechanisms in asthma subgroups”). Asthma mediated via T2-inflammation responds well to therapy with inhaled glucocorticoids (ICS), the main anti-inflammatory therapy used to treat asthma. However, not all patients with asthma respond to inhaled glucocorticoids. Recently, a novel inflammatory type of asthma, so-called T2-low asthma, has been described. In T2-low asthma, the classical features of inflammation mediated via T2-pathways are absent, and these patients are commonly resistant to ICS-therapy. This means that these patients suffer from asthma symptoms, exacerbations and hospitalizations despite standard therapy. Furthermore, use of high doses of ICS or oral corticosteroids (OCS) may even cause or aggravate existing co-morbid diseases such as osteoporosis, obesity, diabetes or glaucoma. We do not know how many people have T2-low asthma, nor the burden it causes to the patients or healthcare system. In order to assess this burden and allocate scarce resources, we need accurate methods to identify these patients. Furthermore, novel treatable traits must be identified to provide personalized treatment and reduce the burden of T2-low asthma.
Our main hypothesis is that there are several clinically relevant and distinct T2-low asthma phenotypes and that these phenotypes associate with specific treatable traits. By using the clinical and survey data collected in WSAS-study and the collaborating studies such as Seinäjoki Adult Asthma Study (SAAS), FinEsS Western Finland, FinnGen and NORDSTAR, we have a toolbox of very large, international epidemiological datasets, including several extensive and detailed clinical phenotyping studies, allowing us to identify clusters of individuals with certain asthma phenotypes and determine their prevalence and risk factors. With this data, we have a unique opportunity to significantly increase the understanding of asthma phenotypes, their mechanisms, and how this disease evolves over time. The project will have high benefit to patients as results will be directly translated to the clinic.
Madeleine Rådinger
Teet Pullerits
Bright Nwaru
Hannu Kankaanranta
About half of all individuals with asthma can be distinguished by the presence of type 2 (T2) inflammation, known as ‘T2-high’ asthma. T2-high asthma involves the pro-inflammatory T2 cytokines interleukin (IL)-4, IL-5, and IL-13. In mild asthma, T2 inflammation appears to be linked to early-onset allergic disease, but the relationship to allergic disease is unclear in those with severe asthma. A new group of effector cells called type 2 innate lymphoid cells (ILC2s) have more recently been identified as mediators of T2 inflammation, independent of allergens or other antigens. These cells are activated by epithelial-derived cytokines (e.g., IL-25, IL-33), which are induced by proteases present not only in allergens, but also in viruses and bacteria. The response to asthma treatment is highly heterogeneous, and those with non-allergic asthma are more likely to report poorly controlled disease. Inhaled corticosteroids, which decrease T2 airway inflammation, are a mainstay of asthma treatment. However, many patients continue to have exacerbations despite treatment, potentially because they have persistent T2-high or T2-low inflammation and other factors driving the immunopathology of disease. The proportion of people with asthma who have signs of severe, poorly controlled disease is much higher than previously thought, markedly worsening quality of life and resulting in an enormous economic burden for society. Poorly controlled asthma includes a range of phenotypes characterized by pathobiology and molecular characteristics in the immune system. Thus, there is a substantial need to increase our understanding of these phenotypes and their underlying causes to improve how we manage this disease.
The overall aim of our research is to identify molecular differences in the immune system among different asthma subgroups that could be targeted for new therapeutic intervention and used as biomarkers in the future.
Specifically, we aim to identify new disease mechanisms in clinically well-characterized severe asthma in a population cohort (WSAS) and severe asthma patient cohort. We also aim to identify new disease-related molecular mechanisms regulating barrier function in primary airway epithelial cells from clinically well-characterized asthma subgroups.
We will integrate epidemiology, clinical asthma phenotyping and mechanistic studies of ex vivo samples from WSAS for molecular phenotyping of human asthma, which gives the current project a unique translational advantage.
Carina Malmhäll
Jenny Calvén
Emma Boberg
Vahid Arabkari
The long-term health consequences of COVID-19 are uncertain, but there might be multi-system effects on survivors. Several studies have been established to define the long-term impact of COVID-19, but most of these are limited by short-term follow-up and participant recruitment is primarily from the hospital. COVID-19 is a disease with varying degrees of severity, including asymptomatic patients, patients with mild symptoms without a need for any healthcare, and those needing intensive care, requiring prolonged mechanical ventilation, or even extracorporeal membrane oxygenation. Investigation into the long-term consequences of COVID-19, covering the future impact of the whole spectrum of disease severity, requires a population-based longitudinal cohort. WSAS-COVID-19 study is a population-representative and clinically focused cohort of adults established to determine long-term respiratory, cardiac, and neurological outcomes in COVID-19 survivors.
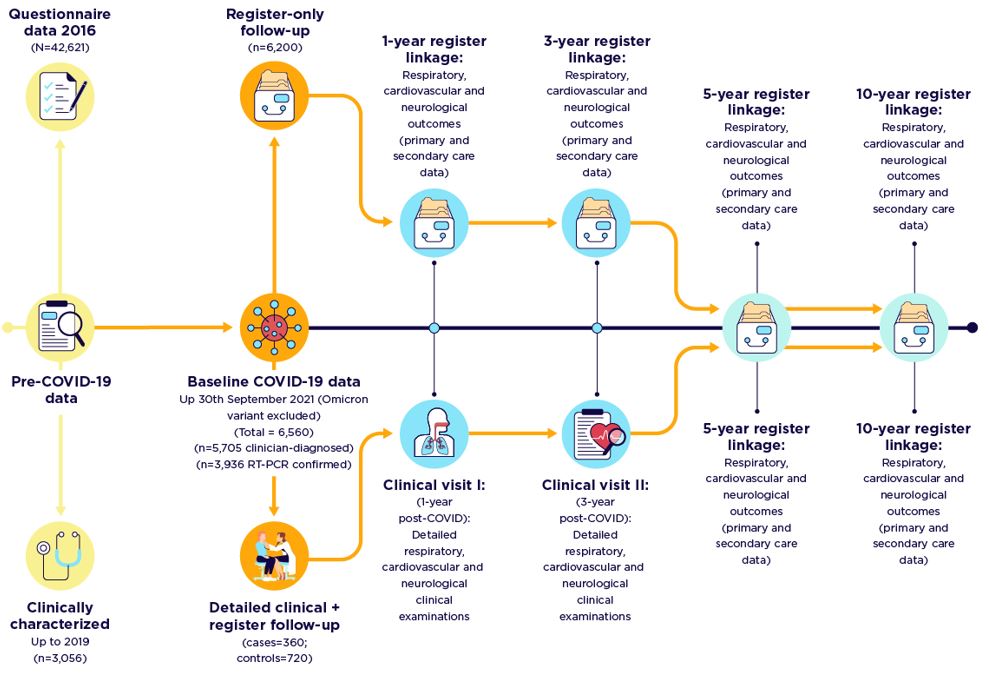
By collecting new clinical and laboratory data to add to already-collected extensive historical questionnaire and clinical data in WSAS, with linkage to health care registers, we aim to define the mid- to long-term clinical prognosis of COVID-19 in a general adult population; determine the cardio-respiratory and neurological morbidity and mortality outcomes; and evaluate the physiological, biochemical, and immunological features related to severity and susceptibility to COVID-19 disease.
Daniil Lisik
Göran Wennergren
Jan Lötvall
Madeleine Rådinger
Fredrik Nyberg
Bright Nwaru
Hannu Kankaanranta

• Hannu Kankaanranta, current principal investigator, Krefting Research Centre, Gothenburg University
• Bright Nwaru, current co-principal investigator, Krefting Research Centre, Gothenburg University
• Linda Ekerljung, researcher and erstwhile principal investigator, Krefting Research Centre, Gothenburg University
• Madeleine Rådinger, chairman and co-principal investigator, Krefting Research Centre, Gothenburg University
• Jan Lötvall, researcher and pioneer principal investigator, Krefting Research Centre, Gothenburg University
• Rani Basna, statistician and database developer and manager, Krefting Research Centre, Gothenburg University
• Lina Rönnebjerg, PhD student, Krefting Research Centre, Gothenburg University
• Guo-Qiang Zhang, PhD student, Krefting Research Centre, Gothenburg University
• Muwada Bashir Awad Bashir, PhD student, Krefting Research Centre, Gothenburg University
• Daniil Lisik, PhD student and database developer and manager, Krefting Research Centre, Gothenburg University
• Selin Özuygur, PhD student, Krefting Research Centre, Gothenburg University
• Eivind Borna, PhD student, Krefting Research Centre, Gothenburg University
• Adina Weisheit, PhD student, Krefting Research Centre, Gothenburg University
• Lotte Edvardsson, research nurse, Krefting Research Centre, Gothenburg University
• Helen Friberg, licensed biomedical analyst, Krefting Research Centre, Gothenburg University
• Louise Olausson, research nurse, Krefting Research Centre, Gothenburg University
• Lina Rönnebjerg, research nurse, Krefting Research Centre, Gothenburg University
• Helén Törnqvist, research laboratory assistant, Krefting Research Centre, Gothenburg University
Krefting Research Centre, Department of Internal Medicine and Clinical Nutrition, Sahlgrenska Academy, Gothenburg University.
Anchor: Collaborators• The OLIN-studies
• Children of Western Sweden (CWS) cohort
• The Nordic EpiLung study
• Seinajoki Adult Asthma Study, Finland
• European Respiratory Society
• AstraZeneca, Sweden
• Thermo Fisher Scientific, Sweden
• The Trøndelag Health Study (The HUNT Study), Norway
• The FinEsS Studies
• Herman Krefting Foundation for Allergy and Asthma Research
• Swedish Research Council
• Swedish Heart-Lung Foundation
• Wallenberg Foundation
• ALF VG-region
We welcome potential collaborators to any of our sub-projects. Please contact the project lead for sub-project you are interested in or write to any of the contact persons below.
Anchor: contactsHannu Kankaanranta
Principal investigator
hannu.kankaanranta@gu.se
Bright Nwaru
Co-principal investigator
+46 31-786 67 18
bright.nwaru@gu.se
Madeleine Rådinger
Chairman and co-principal investigator
+46 31-786 67 13
madeleine.radinger@lungall.gu.se
Nwaru BI, Ekerljung L, Rådinger M, Bjerg A, Mincheva R, Malmhäll C, Axelsson M, Wennergren G, Lotvall J, Lundbäck B. Cohort profile: the West Sweden Asthma Study (WSAS): a multidisciplinary population-based longitudinal study of asthma, allergy and respiratory conditions in adults. BMJ Open. 2019 Jun 19;9(6):e027808. doi: 10.1136/bmjopen-2018-027808. PMID: 31221886; PMCID: PMC6589027.
Borna E, Nwaru BI, Bjerg A, Mincheva R, Rådinger M, Lundbäck B, Ekerljung L. Changes in the prevalence of asthma and respiratory symptoms in western Sweden between 2008 and 2016. Allergy. 2019 Sep;74(9):1703-1715. doi: 10.1111/all.13840. Epub 2019 Jul 1. PMID: 31021427.
Suzuki S, Nwaru BI, Ekerljung L, Sjölander S, Mincheva R, Rönmark EP, Rönmark E, Lundbäck B, Borres MP, Lötvall J. Characterization of sensitization to furry animal allergen components in an adult population. Clin Exp Allergy. 2019 Apr;49(4):495-505. doi: 10.1111/cea.13355. Epub 2019 Feb 27. PMID: 30697845.
Nwaru BI, Suzuki S, Ekerljung L, Sjölander S, Mincheva R, Rönmark EP, Rådinger M, Rönmark E, Borres MP, Lundbäck B, Lötvall J. Furry Animal Allergen Component Sensitization and Clinical Outcomes in Adult Asthma and Rhinitis. J Allergy Clin Immunol Pract. 2019 Apr;7(4):1230-1238.e4. doi: 10.1016/j.jaip.2018.12.018. Epub 2018 Dec 27. PMID: 30594587.
Hedman L, Backman H, Stridsman C, Bosson JA, Lundbäck M, Lindberg A, Rönmark E, Ekerljung L. Association of Electronic Cigarette Use With Smoking Habits, Demographic Factors, and Respiratory Symptoms. JAMA Netw Open. 2018 Jul 6;1(3):e180789. doi: 10.1001/jamanetworkopen.2018.0789. PMID: 30646032; PMCID: PMC6324524.
Mincheva R, Ekerljung L, Bossios A, Lundbäck B, Lötvall J. High prevalence of severe asthma in a large random population study. J Allergy Clin Immunol. 2018 Jun;141(6):2256-2264.e2. doi: 10.1016/j.jaci.2017.07.047. Epub 2017 Sep 20. PMID: 28939411.
Malmhäll C, Johansson K, Winkler C, Alawieh S, Ekerljung L, Rådinger M. Altered miR-155 Expression in Allergic Asthmatic Airways. Scand J Immunol. 2017 Apr;85(4):300-307. doi: 10.1111/sji.12535. PMID: 28199728.
Ekerljung L, Bossios A, Lötvall J, Olin AC, Rönmark E, Wennergren G, Torén K, Lundbäck B. Multi-symptom asthma as an indication of disease severity in epidemiology. Eur Respir J. 2011 Oct;38(4):825-32. doi: 10.1183/09031936.00143710. Epub 2011 Feb 10. PMID: 21310882.
Lötvall J, Ekerljung L, Lundbäck B. Multi-symptom asthma is closely related to nasal blockage, rhinorrhea and symptoms of chronic rhinosinusitis-evidence from the West Sweden Asthma Study. Respir Res. 2010 Nov 26;11(1):163. doi: 10.1186/1465-9921-11-163. PMID: 21110834; PMCID: PMC3004848.
Eriksson J, Ekerljung L, Lötvall J, Pullerits T, Wennergren G, Rönmark E, Torén K, Lundbäck B. Growing up on a farm leads to lifelong protection against allergic rhinitis. Allergy. 2010 Nov;65(11):1397-403. doi: 10.1111/j.1398-9995.2010.02397.x. PMID: 20497148.
Lötvall J, Ekerljung L, Rönmark EP, Wennergren G, Lindén A, Rönmark E, Torén K, Lundbäck B. West Sweden Asthma Study: prevalence trends over the last 18 years argues no recent increase in asthma. Respir Res. 2009 Oct 12;10(1):94. doi: 10.1186/1465-9921-10-94. PMID: 19821983; PMCID: PMC2772988.
Malmhäll C, Weidner J, Rådinger M. MicroRNA-155 expression suggests a sex disparity in innate lymphoid cells at the single-cell level. Cell Mol Immunol. 2020 May;17(5):544-546. doi: 10.1038/s41423-019-0303-4. Epub 2019 Oct 10.PMID: 31601967.
Weidner J, Ekerljung L, Malmhäll C, Miron N, Rådinger M. Circulating microRNAs correlate to clinical parameters in individuals with allergic and non-allergic asthma. Respir Res. 2020 May 7;21(1):107. doi: 10.1186/s12931-020-01351-x.PMID: 32381094
Anchor: links